- Research
High Rate Electrochemical Reduction of Carbon Monoxide to Ethylene using Cu-Nanoparticle-Based Gas Diffusion Electrodes
Han, L., Zhou, W., Xiang, C. High Rate Electrochemical Reduction of Carbon Monoxide to Ethylene using Cu-Nanoparticle-Based Gas Diffusion Electrodes. ACS Energy Letters, DOI: 10.1021/acsenergylett.8b00164 (2018)
Scientific Achievement
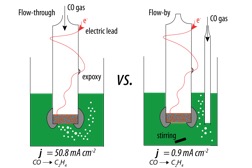
This work achieved a high geometric partial current density of 50.8 mA cm-2 for electrochemical CO reduction to C2H4 at -0.85 V vs. RHE in a flow-through gas diffusion electrode (GDE).
Significance & impact
This work demonstrates that direct gas feed configurations, such as flow-through GDEs, provide a unique electrode structure for efficient and stable electrochemical reactions without mass transport limitations, especially for gases with low solubility in aqueous solutions, such as CO, CO2 and N2.
Research Details
•Cu-nanoparticle-based GDEs were fabricated by applying a mixture of carbon powders, copper acetate aqueous solution, and Teflon onto a Cu gauze substrate. The catalyst-coated substrate was air-dried, mechanically pressed and subsequently annealed under forming gas to produce GDEs.
•In the flow through configuration, a high partial current density of 50.8 mA cm-2 and a Faraday efficiency of 17.8% for COR to C2H4 were achieved in 10 M KOH at -15oC, while in the flow-by configuration, the partial current density was limited to < 1 mA cm-2.
Two distinctive GDE configurations were constructed and tested.
Reprinted with permission from Han, L., Zhou, W., Xiang, C. High Rate Electrochemical Reduction of Carbon Monoxide to Ethylene using Cu-Nanoparticle-Based Gas Diffusion Electrodes. ACS Energy Letters, DOI: 10.1021/acsenergylett.8b00164 (2018).